Phosphorous acid
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphonic acid
| |||
| Systematic IUPAC name
Phosphorous acid | |||
| Other names
Dihydroxyphosphine oxide
Dihydroxy(oxo)-λ5-phosphane | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.682 | ||
| EC Number |
| ||
| 1619 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2834 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H3PO3 | |||
| Molar mass | 81.99 g/mol | ||
| Appearance | white solid deliquescent | ||
| Density | 1.651 g/cm3 (21 °C) | ||
| Melting point | 73.6 °C (164.5 °F; 346.8 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) (decomposes) | ||
| 310 g/100 mL | |||
| Solubility | soluble in ethanol | ||
| Acidity (pKa) | 1.3, 6.7 | ||
| −42.5·10−6 cm3/mol | |||
| Structure | |||
| pseudo-tetrahedral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
skin irritant | ||
| GHS labelling:[1] | |||
 
| |||
| Danger | |||
| H302, H314 | |||
| P260, P264, P270, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | Sigma-Aldrich | ||
| Related compounds | |||
Related compounds
|
H3PO4 (i.e., PO(OH)3) H3PO2 (i.e., H2PO(OH)) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Phosphorous acid (or phosphonic acid) is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.
Nomenclature and tautomerism
[edit]
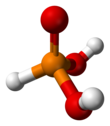
Solid HP(O)(OH)2 has tetrahedral geometry about the central phosphorus atom, with a P−H bond of 132 pm, one P=O double bond of 148 pm and two longer P−OH single bonds of 154 pm. In common with other phosphorus oxides with P−H bonds (e.g.hypophosphorous acid and dialkyl phosphites),[2] it exists in equilibrium with an extremely minor tautomer P(OH)3. (In contrast, arsenous acid's major tautomer is the trihydroxy form.) IUPAC recommends that the trihydroxy form P(OH)3 be called phosphorous acid, and the dihydroxy form HP(O)(OH)2 phosphonic acid.[3] Only the reduced phosphorus compounds are spelled with an "-ous" ending.
- PIII(OH)3 ⇌ HPV(O)(OH)2 K = 1010.3 (25°C, aqueous)[4]
Preparation
[edit]On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam:[5]
- PCl3 + 3 H2O → HPO(OH)2 + 3 HCl
HPO(OH)2 could be produced by the hydrolysis of phosphorus trioxide:
- P4O6 + 6 H2O → 4 HPO(OH)2
Reactions
[edit]Acid–base properties
[edit]Phosphorous acid has a pKa in the range 1.26–1.3.[6][7]
- HP(O)(OH)2 → HP(O)2(OH)− + H+ pKa = 1.3
It is a diprotic acid, the hydrogenphosphite ion, HP(O)2(OH)− is a weak acid:
- HP(O)2(OH)− → HPO2−3 + H+ pKa = 6.7
The conjugate base HP(O)2(OH)− is called hydrogen phosphite, and the second conjugate base, HPO2−3, is the phosphite ion.[8] (Note that the IUPAC recommendations are hydrogen phosphonate and phosphonate respectively).
The hydrogen atom bonded directly to the phosphorus atom is not readily ionizable. Chemistry examinations often test students' appreciation of the fact that not all three hydrogen atoms are acidic under aqueous conditions, in contrast with H3PO4.
Redox properties
[edit]On heating at 200 °C, phosphorous acid disproportionates to phosphoric acid and phosphine:[9]
- 4 H3PO3 → 3 H3PO4 + PH3
This reaction is used for laboratory-scale preparations of PH3.
Phosphorous acid slowly oxidizes in air to phosphoric acid.[5]
Both phosphorous acid and its deprotonated forms are good reducing agents, although not necessarily quick to react. They are oxidized to phosphoric acid or its salts. It reduces solutions of noble metal cations to the metals. When phosphorous acid is treated with a cold solution of mercuric chloride, a white precipitate of mercurous chloride forms:
- H3PO3 + 2 HgCl2 + H2O → Hg2Cl2 + H3PO4 + 2 HCl
Mercurous chloride is reduced further by phosphorous acid to mercury on heating or on standing:
- H3PO3 + Hg2Cl2 + H2O → 2 Hg + H3PO4 + 2 HCl
As a ligand
[edit]
Upon treatment with metals of d6 configuration, phosphorous acid is known to coordinate as the otherwise rare P(OH)3 tautomer. Examples include Mo(CO)5(P(OH)3) and [Ru(NH3)4(H2O)(P(OH)3)]2+.[10][11]
Heating a mixture of potassium tetrachloroplatinate and phosphorous acid gives the luminescent salt potassium diplatinum(II) tetrakispyrophosphite:[12]
- 2 K2PtCl4 + 8 H3PO3 → K4[Pt2(HO2POPO2H)4] + 8 HCl + 4 H2O
Uses
[edit]The most important use of phosphorous acid (phosphonic acid) is the production of basic lead phosphite, which is a stabilizer in PVC and related chlorinated polymers.[5]
It is used in the production of basic lead phosphonate PVC stabilizer, aminomethylene phosphonic acid and hydroxyethane diphosphonic acid. It is also used as a strong reducing agent and in the production of synthetic fibres, organophosphorus pesticides, and the highly efficient water treatment agent ATMP.
Ferrous materials, including steel, may be somewhat protected by promoting oxidation ("rust") and then converting the oxidation to a metalophosphate by using phosphoric acid and further protected by surface coating. (See: Passivation (chemistry)).
Organic derivatives
[edit]The IUPAC (mostly organic) name is phosphonic acid. This nomenclature is commonly reserved for substituted derivatives, that is, organic group bonded to phosphorus, not simply an ester. For example, (CH3)PO(OH)2 is "methylphosphonic acid", which may of course form "methylphosphonate" esters.
References
[edit]- ^ "Phosphorous acid". pubchem.ncbi.nlm.nih.gov.
- ^ Janesko, Benjamin G.; Fisher, Henry C.; Bridle, Mark J.; Montchamp, Jean-Luc (2015-09-29). "P(═O)H to P–OH Tautomerism: A Theoretical and Experimental Study". The Journal of Organic Chemistry. 80 (20). American Chemical Society (ACS): 10025–10032. doi:10.1021/acs.joc.5b01618. ISSN 0022-3263. PMID 26372089.
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. Electronic version..
- ^ Guthrie, J. Peter (1979). "Tautomerization Equilibria for Phosphorous Acid and its Ethyl Esters, Free Energies of Formation of Phosphorous and Phosphonic Acids and their Ethyl Esters, and p Ka Values for Ionization of the P—H Bond in Phosphonic Acid and Phosphonic Esters". Canadian Journal of Chemistry. 57 (2): 236–239. doi:10.1139/v79-039.
- ^ a b c Bettermann, Gerhard; Krause, Werner; Riess, Gerhard; Hofmann, Thomas (2000). "Phosphorus Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_527. ISBN 978-3527306732..
- ^ Larson, John W.; Pippin, Margaret (1989). "Thermodynamics of ionization of hypophosphorous and phosphorous acids. Substituent effects on second row oxy acids". Polyhedron. 8 (4): 527–530. doi:10.1016/S0277-5387(00)80751-2.
- ^ CRC Handbook of Chemistry and Physics (87th ed.). pp. 8–42.
- ^ Novosad, Josef (1994). Encyclopedia of Inorganic Chemistry. John Wiley and Sons. ISBN 0-471-93620-0.
- ^ Gokhale, S. D.; Jolly, W. L. (1967). "Phosphine". Inorganic Syntheses. Vol. 9. pp. 56–58. doi:10.1002/9780470132401.ch17. ISBN 9780470132401.
- ^ a b Xi, Chanjuan; Liu, Yuzhou; Lai, Chunbo; Zhou, Lishan (2004). "Synthesis of molybdenum complex with novel P(OH)3 ligand based on the one-pot reaction of Mo(CO)6 with HP(O)(OEt)2 and water". Inorganic Chemistry Communications. 7 (11): 1202–1204. doi:10.1016/j.inoche.2004.09.012.
- ^ Sernaglia, R. L.; Franco, D. W. (2005). "The ruthenium(II) center and the phosphite-phosphonate tautomeric equilibrium". Inorg. Chem. 28 (18): 3485–3489. doi:10.1021/ic00317a018.
- ^ Alexander, K. A.; Bryan, S. A.; Dickson, M. K.; Hedden, D.; Roundhill (2007). "Potassium Tetrakis[Dihydrogen Diphosphito(2–)]Diplatinate(II)". Inorganic Syntheses. Vol. 24. pp. 211–213. doi:10.1002/9780470132555.ch61. ISBN 9780470132555.
Further reading
[edit]- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- Corbridge., D. E. C. (1995). Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 0-444-89307-5.
- Lee, J.D. (3 January 2008). Concise Inorganic Chemistry. Oxford University Press. ISBN 978-81-265-1554-7.